Introduction to Chemistry - Antacid and Vinegar
Introduce your students to core chemistry concepts as well as data collection and analysis with a quick demonstration using common household chemicals. This classic demonstration of a chemical reaction is quantified and enhanced with pH, temperature and conductivity measurements using the Advanced Chemistry Sensor. This quick demonstration will help the students think about some of the concepts that they will see throughout the year and the types of measurements that they can make to explore those concepts.
Demonstration
- Add ~60 mL of water and ~60 mL of household vinegar to a 150-mL beaker.
- Ask the students what the pH should be. Most will assume the pH is low since vinegar is an acid.
- Put the pH, Temperature, and Conductivity Probes in the beaker. Then, create a graph of pH vs. Time and a digits display of pH.
- Ask students to predict what will happen to the pH once the antacid tablet is added.
pH vs. Time
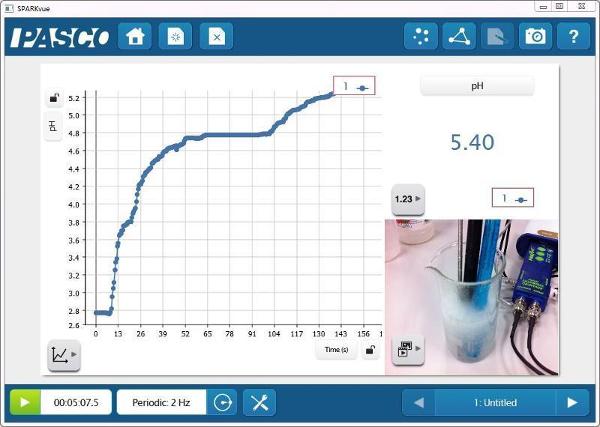
Since the Temperature and Conductivity Probes were also in the solution, there are more opportunities for measurement-based explorations of the system.
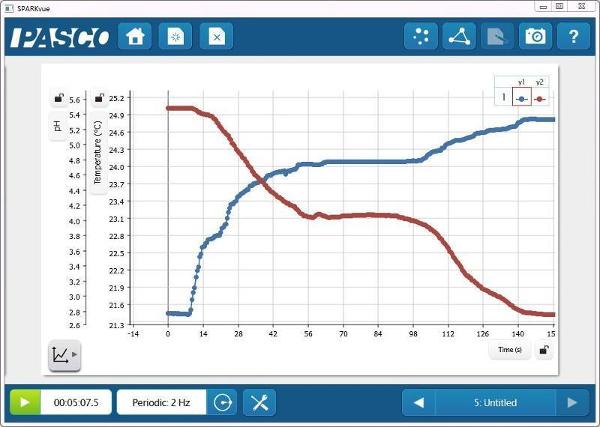
- Ask students to predict the temperature change as the reaction proceeds.
- Ask students to predict the conductivity change as the reaction proceeds.
pH and Temperature vs. Time
pH, Temperature, and Conductivity vs. Time
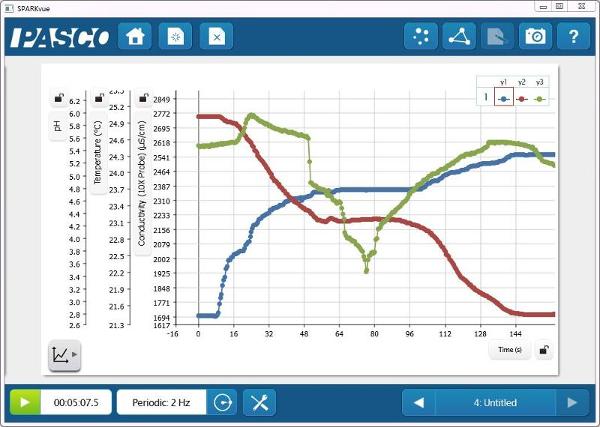
The reaction of antacid and vinegar provides a nice visual indication that a chemical change is taking place. By monitoring the reaction with sensors, core chemistry concepts, including acids and bases, electrolytes, and thermochemistry can also be introduced.
Student Extension
Time permitting, have the students perform other experiments with beakers of “unknowns” to see of visual observations match sensor data.
- The students can mix one “unknown” beaker containing 100 mL of water with blue food coloring and another beaker with 100 mL of yellow food coloring.
There will be a color change to green, but the measurement data will not indicate a chemical change
- The students can mix one “unknown” beaker containing 100 mL of 0.1 M HCl and another beaker containing 100 mL of 0.1 M NaOH
There will be no obvious visual indication of a chemical reaction, but the measurements will indicate that a change has taken place.
